Beginners Guide to ISE Measurement. Chapter 5.
REFERENCE ELECTRODES
In order to measure the change in potential difference across the ion-selective membrane as the ionic concentration changes, it is necessary to include in the circuit a stable reference voltage which acts as a half-cell from which to measure the relative deviations.
a) The Silver / Silver Chloride Single Junction Reference Electrode.
The most common and simplest reference system is the silver / silver chloride single junction reference electrode. This generally consists of a cylindrical glass or plastic tube containing a 4 Molar solution of KCl saturated with AgCl. The lower end is sealed with a porous ceramic frit which allows the slow passage of the internal filling solution and forms the liquid junction with the external test solution. Dipping into the filling solution is a silver wire coated with a layer of silver chloride (it is chloridised) which is joined to a low-noise cable which connects to the measuring system.
In electrochemical terms, the half-cell can be represented by:
Ag / AgCl (Satd), KCL (Satd)
and the electrode reaction is:
AgCl (s) + e- = Ag (s) + Cl-
The electrode potential for this half-cell is + 0.2046 V relative to the Standard Hydrogen Electrode at 25°C
b) Double Junction Reference Electrodes.
One problem with reference electrodes is that, in order to ensure a stable voltage, it is necessary to maintain a steady flow of electrolyte through the porous frit. Thus there is a gradual contamination of the test solution with electrolyte ions. This can cause problems when trying to measure low levels of K, Cl, or Ag, or when using other ISEs with which these elements may cause interference problems. In order to overcome this difficulty the double junction reference electrode was developed. In this case the silver / silver chloride cell described above forms the inner element and this is inserted into an outer tube containing a different electrolyte which is then in contact with the outer test solution through a second porous frit. The outer filling solution is said to form a "salt bridge" between the inner reference system and the test solution and is chosen so that it does not contaminate the test solution with any ions which would effect the analysis.
Commonly used outer filling solutions are:
potassium nitrate - for Br, Cd, Cl, Cu, CN, I, Pb, Hg, Ag, S, SCN.
sodium chloride - for K,
ammonium sulphate - for N03,
magnesium sulphate - for NH4,
Double junction reference electrodes are named after their outer filling solutions.
Note Added in 2017: In recent years several suppliers have replaced all these DJ electrodes by one containing Lithium Acetate in the outer chamber which can be used with any ISE. This is far more versatile because neither ion interferes with any ISE and they have very similar mobilities; giving a nearly "equitransferent" solution which is less prone to variable liquid junction potentials.
One disadvantage with double junction reference electrodes is that they introduce an extra interface between two electrolytes and thus give the opportunity for an extra liquid junction potential to develop.
c) Liquid Junction Potentials.
It must be noted that the standard voltage given by a reference electrode is only correct if there is no additional voltage supplied by a liquid junction potential formed at the porous plug between the filling solution and the external test solution. Liquid junction potentials can appear whenever two dissimilar electrolytes come into contact. At this junction, a potential difference will develop as a result of the tendency of the smaller and faster ions to move across the boundary more quickly than those of lower mobility. These potentials are difficult to reproduce, tend to be unstable, and are seldom known with any accuracy; so steps must be taken to minimise them. Using 4 Molar KCL as the inner filling solution has the advantage that the K+ and Cl- ions have nearly equal mobilities and hence form an equi-transferrent solution. Also, in the single junction electrodes, the electrolyte concentration is much higher than that of the sample solution thus ensuring that the major portion of the current is carried by these ions. A third factor in minimising the junction potential is the fact that there is a small but constant flow of electrolyte out from the electrode thus inhibiting any back-diffusion of sample ions - although this is less important with modern gel electrolytes.
As indicated above, all these problems are doubled when double junction reference electrodes are used and an additional problem arises in the case of the last three listed above (Sodium Chloride, Ammonium Sulphate, Magnesium Sulphate) because the filling solutions are not equi-transferrent and hence have a stronger tendency to form liquid junction potentials. It must be noted here that Nico2000 Ltd have recently introduced a novel Lithium Acetate reference electrode which overcomes most of these problems and can be used with all the ELIT range of ISEs. This is because it contains ions which are very nearly equi-tranferrent and which do not interfere with any of the commonly used ISEs.
It must be noted that the E0 factor in the Nernst equation is the sum of all the liquid junction potentials present in the system and any variation in this during analyses can be a major source of potential drift and error in measurements.
d) Combination Electrodes
The majority of pH electrodes are produced in the form of combination electrodes in which the reference system is housed in the same cylindrical body as the sensor head. This produces a simple, compact unit for immersing in the test solution and has the added advantage that the two cells are in close proximity (with the reference cell normally completely surrounding the sensor element) - thus minimising the effect of any stray electrostatic fields or any inhomogeneity in the test solution. The main disadvantage of this arrangement is the fact that it is the reference element which is the most likely to cause problems or fail, long before the ISE head does, but the whole unit has to be replaced when failure does occur.
In contrast to pH electrodes, some ISEs are produced as mono-electrodes for use with separate reference systems. One reason for this is because ISE membranes have a far lower impedance than pH sensors and are less susceptible to stray electrostatic fields. Thus it is not necessary to screen the sensor head by surrounding it with the reference system. More importantly, the membranes and internal construction of ISEs are generally far more expensive than pH sensors and it is much more cost-effective to have separate units in which the reference system can be replaced independently from the ISE.
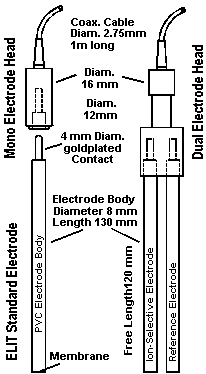
e) Multiple Electrode Heads: Separable Combinations.
A new concept for combination electrodes has recently been introduced. Both the ISEs and the reference electrodes are made in the form of 8mm diameter tubes fitted with a gold plated plug-in connector. These can be inserted separately into special multiple electrode heads which are fitted with the cables and connectors for attaching to the measuring system. The rigid plastic head ensures that the ISE and reference system remain firmly linked together at a regular distance apart during operation, but either one can easily be replaced in the event of failure or need to change the analysis. Moreover, the replacement electrodes are relatively inexpensive compared to conventional electrodes because they do not incorporate the expensive low-noise cables.
The ELIT Electrode Head System
A practical and cost effective way to combine Ion Selective and Reference Electrodes
ELIT Electrode Heads are manufactured
from a robust plastic material and fitted with low noise cables and
connectors which are compatible with any standard mV/pH/ion meter.
The standard version, for use with an ELIT Ion Analyser / Computer Interface, has a BNC plug, but DIN, US or S7 versions are available if required.
The sockets on the head and the pins on the plug-in electrodes are gold plated to assure good contact.
Advantages of this 'electrode combination'
over conventional combination electrodes:
- Use of one reference electrode for several ion-selective electrodes.
- Replacement of a defective reference system without sacrificing the more expensive ISE.
- Expensive low-noise cable and connector are attached to the re-usable head and do not need to be replaced if the ISE becomes defective.
- ISE is less expensive than conventional types with cable & connectors permanently attached.
- ISE can be stored dry and the RE wet.
- Increased distance between the ISE and the reference system reduces electrical interference and increases the precision of measurement.