Beginners Guide to ISE Measurement. Chapter 4.
TYPES OF ION SELECTIVE ELECTRODES
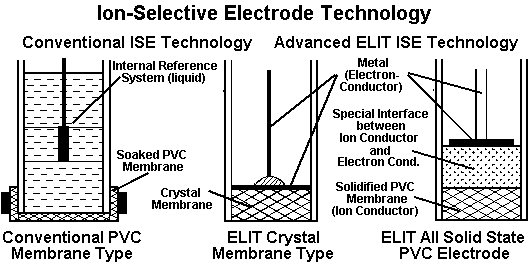
Also see: ELIT Ion-Selective Electrodes and How ISEs Work.
a) General Discussion
Ion selective electrodes come in various shapes and sizes. Each manufacturer has it’s own distinctive features, but very few give details of the internal construction of the electrode or composition of the ion-selective membranes. These are the most important factors which control the performance of the electrode, and are often kept as closely guarded trade secrets. Nevertheless, there are certain features that are common to all. All consist of a cylindrical tube, generally made of a plastic material, between 5 and 15 mm in diameter and 5 to 10 cm long. An ion-selective membrane is fixed at one end so that the external solution can only come into contact with the outer surface, and the other end is fitted with a low noise cable or gold plated pin for connection to the millivolt measuring device. In some cases the internal connections are completed by a liquid or gel electrolyte, in others by an all-solid-state system.
Ion-selective membranes are currently only available for a limited number of commonly occurring ionic species. Examination of manufacturer’s catalogues reveals that the most common are:
CATIONS: Ammonium (NH4+), Barium (Ba++), Calcium (Ca++), Cadmium (Cd++),
Copper (Cu++), Lead (Pb++), Mercury (Hg++), Potassium (K+), Sodium (Na+), Silver (Ag+).
ANIONS: Bromide (Br-), Chloride (Cl-), Cyanide (CN-), Fluoride (F-),
Iodide (I-), Nitrate (NO3-), Nitrite (NO2-), Perchlorate (ClO4-), Sulphide (S-), Thiocyanate (SCN-).
The manner in which these different membranes select and transport the particular ions is highly variable and in many cases highly complex. It is far beyond the scope of this work to explain in detail the exact mechanism for each ion. Moreover, it is not necessary for the analyst to understand these mechanisms in order to use the electrodes satisfactorily. Nevertheless, it may be of interest to the general reader to give some indication of these processes. There are two main types of membrane material, one based on a solid crystal matrix, either a single crystal or a polycrystalline compressed pellet, and one based on a plastic or rubber film impregnated with a complex organic molecule which acts as an ion-carrier. The development of these organic membranes was based on biological research which revealed that some antibiotics and vitamins can induce cationic permeation through cell membranes. One example of each membrane type is described below as an illustration of the range of technologies employed.
b) Crystal-Membrane Electrodes e.g. Fluoride.
The Fluoride electrode is a typical example of the first type. Here the membrane consists of a single lanthanum fluoride crystal which has been doped with europium fluoride to reduce the bulk resistivity of the crystal. It is 100% selective for F- ions and is only interfered with by OH- which reacts with the lanthanum to form lanthanum hydroxide, with the consequent release of extra F- ions. This interference can be eliminated by adding a pH buffer to the samples to keep the pH in the range 4 to 8 and hence ensure a low OH- concentration in the solutions.
c) Impregnated-PVC-Membrane Electrodes e.g. Potassium.
The Potassium electrode was one of the earliest developed and simplest examples of the second type. The membrane is usually in the form of a thin disc of PVC impregnated with the macrocyclic antibiotic valinomycin. This compound has a hexagonal ring structure with an internal cavity which is almost exactly the same size as the diameter of the K+ ion. Thus it can form complexes with this ion and preferentially conducts it across the membrane. Unfortunately it is not 100% selective and can also conduct small numbers of sodium and ammonium ions. Thus these can cause errors in the potassium determination if they are present in high concentrations. The majority of other ISEs suffer from similar limitations (see later section on ‘interference’).
Several other complex processes are employed in ion-selective membranes and details of these can be found in specialist electrochemistry textbooks and in catalogues from manufacturers of "ionophores".
d) Care and Maintenance of ISEs.
When handling ISEs, care should be taken to avoid damaging the membrane surface. If the electrodes are in frequent use then they can simply be left hanging in the electrode holder with the membrane surface open to the air but protected by a clean dry beaker. For prolonged storage in a cupboard or drawer, the membrane should be protected by covering with the rubber or plastic cap which is normally provided with the electrode. After extensive use the membranes may become coated with a deposit or scoured with fine scratches which may cause a slow or reduced response (low slope) or unstable readings.
Crystal membranes can be regenerated by washing with alcohol and/or gently polishing with fine emery paper to remove any deposit or discoloration, then thoroughly washing with de-ionised water to remove any debris. After this, they may require soaking in the concentrated standard solution for several hours before a stable reading can be re-established. It must be noted, however, that prolonged immersion of crystal membranes in aqueous solutions will eventually cause a build up of oxidation products on the membrane surface and thus inhibit performance and shorten the active life. Conversely, PVC membranes should not even be touched, let alone polished, and can be often be regenerated by prolonged (several days) soaking in the standard solution, after removing any deposit with a fine jet of water, or rinsing in alcohol.
NEXT PAGE | PREVIOUS PAGE | BACK TO CONTENTS LIST