|
NEXT PAGE |
PREVIOUS PAGE |
BACK TO CONTENTS LIST
| GO TO WWW.NICO2000.NET |
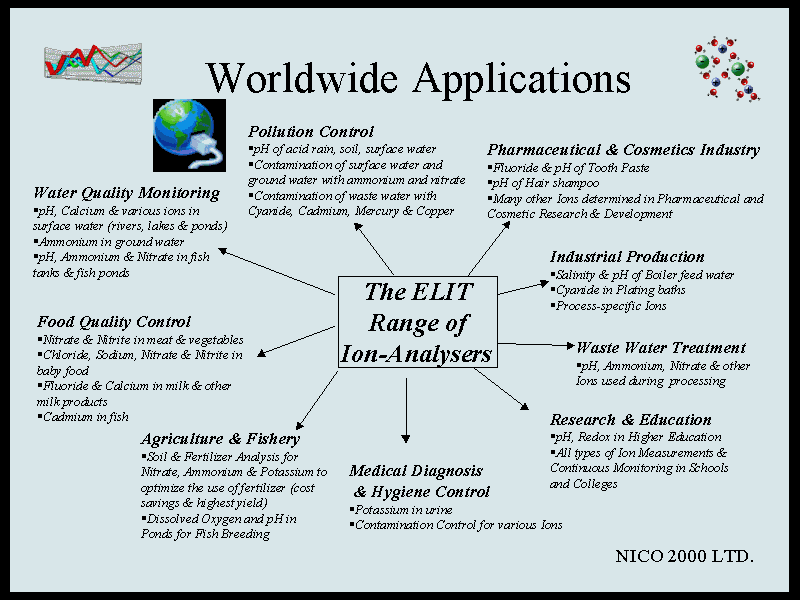
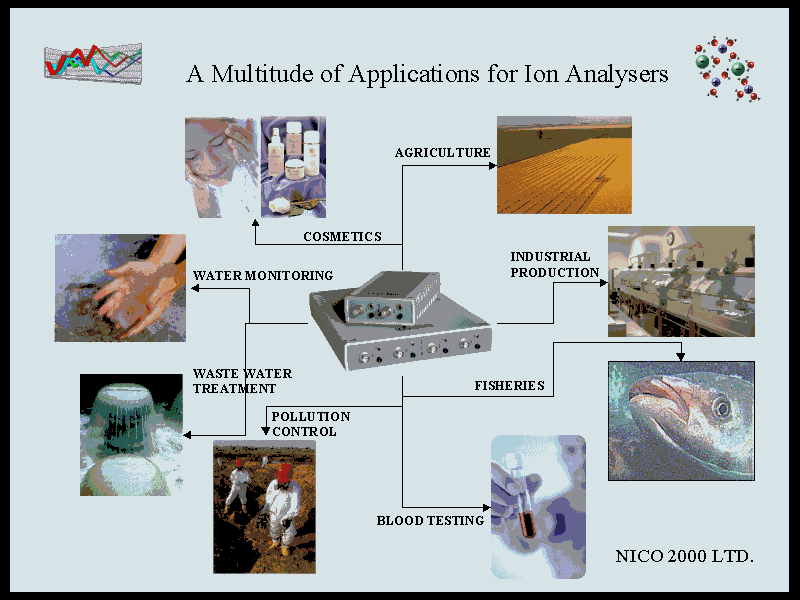
Beginners Guide to ISE Measurement, Chapter 2. a) Applications Ion-selective electrodes are used in a wide variety of applications for determining the concentrations of various ions in aqueous solutions. The following is a list of some of the main areas in which ISEs have been used. Pollution Monitoring: CN, F, S, Cl, NO3 etc., in effluents, and natural waters. Agriculture: NO3, Cl, NH4, K, Ca, I, CN in soils, plant material, fertilisers and feedstuffs. Food Processing: NO3, NO2 in meat preservatives. Salt content of meat, fish, dairy products, fruit juices, brewing solutions. F in drinking water and other drinks. Ca in dairy products and beer. K in fruit juices and wine making. Corrosive effect of NO3 in canned foods. Detergent Manufacture: Ca, Ba, F for studying effects on water quality. Paper Manufacture: S and Cl in pulping and recovery-cycle liquors. Explosives: F, Cl, NO3 in explosive materials and combustion products. Electroplating: F and Cl in etching baths; S in anodising baths. Biomedical Laboratories: Ca, K, Cl in body fluids (blood, plasma, serum, sweat). F in skeletal and dental studies. Education and Research: Wide range of applications. b) Advantages. 1) When compared to many other analytical techniques, Ion-Selective Electrodes are relatively inexpensive and simple to use and have an extremely wide range of applications and wide concentration range. 2) The most recent plastic-bodied all-solid-state or gel-filled models are very robust and durable and ideal for use in either field or laboratory environments. 3) Under the most favourable conditions, when measuring ions in relatively dilute aqueous solutions and where interfering ions are not a problem, they can be used very rapidly and easily (e.g. simply dipping in lakes or rivers, dangling from a bridge or dragging behind a boat). 4) They are particularly useful in applications where only an order of magnitude concentration is required, or it is only necessary to know that a particular ion is below a certain concentration level. 5) They are invaluable for the continuous monitoring of changes in concentration: e.g. in potentiometric titrations or monitoring the uptake of nutrients, or the consumption of reagents. 6) They are particularly useful in biological/medical applications because they measure the activity of the ion directly, rather than the concentration. 7) In applications where interfering ions, pH levels, or high concentrations are a problem, then many manufacturers can supply a library of specialised experimental methods and special reagents to overcome many of these difficulties. 8) With careful use, frequent calibration, and an awareness of the limitations, they can achieve accuracy and precision levels of ± 2 or 3% for some ions and thus compare favourably with analytical techniques which require far more complex and expensive instrumentation. 9) ISEs are one of the few techniques which can measure both positive and negative ions. 10) They are unaffected by sample colour or turbidity. 11) ISEs can be used in aqueous solutions over a wide temperature range. Crystal membranes can operate in the range 0°C to 80°C and plastic membranes from 0°C to 50°C. |